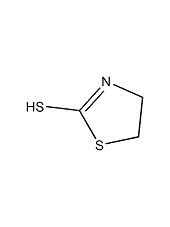
Structural formula
| Business number | 02BA |
|---|---|
| Molecular formula | C3H3NS2 |
| Molecular weight | 119.21 |
| label |
2-mercapto-2-thiazoline, 2-Thiazoline-2-thiol, 1,3-Thiazolidine-2-thione |
Numbering system
CAS number:96-53-7
MDL number:MFCD00126013
EINECS number:202-512-1
RTECS number:XJ6122000
BRN number:106332
PubChem number:24897083
Physical property data
1. Properties: light brown or white solid with foul odor.
2. Density (g/mL, 20?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 105-107
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
7. p>
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg,ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Dissolved in CH2 Cl2 and most polar organic solvents. Usually used in CH2Cl2.
Toxicological data
1. Acute toxicity:
Rat oral LD50: 300mg/kg;
Rat peritoneal cavity LDL0: 500mg/kg;
Small Rat oral LDL0: 710mg/kg;
Mouse peritoneal cavity LD50: 200mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 30.90
2. Molar volume (cm3/mol): 84.7
3. Isotonic specific volume (90.2K ): 233.0
4. Surface tension (dyne/cm): 57.2
5. Polarizability (10-24cm3): 12.25
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 69.4
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 71.2
10.Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine Number of stereocenters of chemical bonds: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides.
2. Stable to air and moisture. Since its derivatives display a variety of biological activities, handling and use in a fume hood is recommended.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Prepared by aminoethyl sulfate and carbon disulfide under base catalysis[1].
Purpose
1. Used as acid bright copper plating additive and pharmaceutical intermediate.
2. The role of 1,3-thiazolidine-2-thione in organic synthesis is mainly expressed through the amide derivatives generated by its reaction with carboxylic acid or acid halide. When 1,3-thiazolidine-2-thione generates amide derivatives, the reactivity of the acyl group is activated. For example: amides can be selectively reduced by DIBAL-H to produce the corresponding aldehydes, or reduced by NaBH4 in the presence of other ester groups or amides to produce the corresponding alcohols[2]. But these functions have been covered by other reaction reagents and conditions. Currently, 1,3-thiazolidine-2-thioneamide derivatives are mainly used as selective acylating reagents for amino and hydroxyl groups.
1,3-thiazolidine-2-thione and carboxylic acid can form amides in the presence of the condensation reagent DCC, or can also form amides with acid halides in the presence of triethylamine and DMAP. The generated amide derivatives and amino compounds can be left at room temperature under neutral conditions or heated together to make 1,3-thiazolidine-2-thione leave and generate new amides. This reaction is also often used in the reaction of macrocyclic compounds, generally giving very satisfactory yields [3]. If there are different functional groups, it also shows a high degree of chemical selectivity (Formula 1~Formula 3)[4,5].
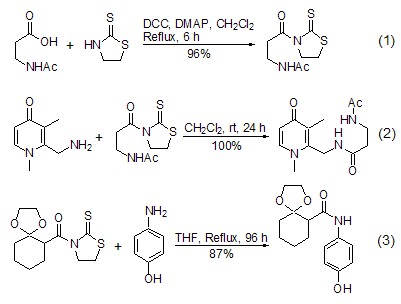
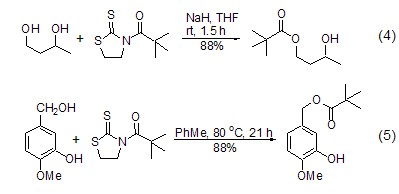
1,3-thiazolidine- The corresponding ester [6~8] can be easily obtained by co-heating 2-thione amide derivatives with alcohol. A high degree of regioselectivity can be achieved using this method when different alcohols are present simultaneously. The selectivity is determined on the one hand by the steric hindrance of the acyl segment in the amide derivative, with tert-pentanoyl giving the best results. On the other hand, it depends on the type of hydroxyl group, and the order of activity is primary alcohol > secondary alcohol > phenol (Formula 4, Formula 5) [7,8].
extended-reading:https://www.newtopchem.com/archives/category/products/page/128extended-reading:https://www.newtopchem.com/archives/44286extended-reading:https://www.bdmaee.net/non-silicone-silicone-oil/extended-reading:https://www.newtopchem.com/archives/1748extended-reading:https://www.bdmaee.net/nt-cat-mb20-catalyst-cas-68007-43-3-newtopchem/extended-reading:https://www.morpholine.org/catalyst-dabco-pt303-composite-tertiary-amine-catalyst-dabco-pt303/extended-reading:https://www.cyclohexylamine.net/dimethyltin-dioctanoate-cas-68928-76-7/extended-reading:https://www.bdmaee.net/nt-cat-la-404-catalyst-cas1066-33-4-newtopchem/extended-reading:https://www.newtopchem.com/archives/44854extended-reading:https://www.bdmaee.net/toyocat-dmi-gel-catalyst-tosoh/