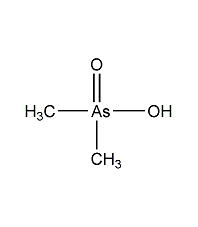
Structural formula
| Business number | 01JV |
|---|---|
| Molecular formula | C2H7AsO2 |
| Molecular weight | 138.00 |
| label |
Dimethylarsinic acid, herbicide |
Numbering system
CAS number:75-60-5
MDL number:MFCD00002095
EINECS number:200-883-4
RTECS number:CH7525000
BRN number:1736965
PubChem number:24892235
Physical property data
1. Characteristics: colorless crystals.
2. Density (g/mL,25/4?): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):192 -198?
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Saturated vapor pressure (kPa,60ºC): Unsure
13. Heat of combustion (KJ/mol): Unsure
14. Critical temperature (ºC): Unsure
15. Critical pressure (KPa): Unsure
16. Oil and water (octanol/Logarial value of partition coefficient for water: Uncertain
17. Explosion limit (%,V/V): Unsure
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:25Solubility in water at ? is200g/100g,Soluble in lower alcohols.
Toxicological data
1, acute toxicity: rat oral administrationLD50?644mg/kg; rat by inhalationLCLo?2600mg/m3/2H .
Ecological data
It is extremely harmful to water, even in small amounts, to fish and floating fish in water bodies Creatures are poisonous
It is highly toxic to organic matter in the water and fish.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA):37.3
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 62
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Covalent Number of key units: 1
Properties and stability
Reacts with moisture, air, and oxides.
Storage method
Store airtightly,Store in a cool, dark place Dry warehouse. Avoid contact with moisture, water, and air.
Synthesis method
Reduced by disodium methylarsinate with sulfur dioxide, and then under alkaline conditions It reacts with methyl chloride to form sodium dimethylarsinate, and then it is treated with hydrochloric acid.
Purpose
Used to control weeds in non-crop fields,Dosage10?15kg/ha
extended-reading:https://www.newtopchem.com/archives/45041extended-reading:https://www.newtopchem.com/archives/44233extended-reading:https://www.bdmaee.net/cas-1704-62-7/extended-reading:https://www.bdmaee.net/dioctyl-dimaleate-di-n-octyl-tin/extended-reading:https://www.newtopchem.com/archives/40372extended-reading:https://www.cyclohexylamine.net/catalyst-1028-polyurethane-catalyst-1028/extended-reading:https://www.bdmaee.net/toyocat-np-catalyst-tosoh/extended-reading:https://www.bdmaee.net/niax-k-zero-3000-trimer-catalyst-momentive/extended-reading:https://www.bdmaee.net/polyurethane-reaction-inhibitor/extended-reading:https://www.bdmaee.net/pc-cat-np30-catalyst-trisdimethylaminomethylphenol/