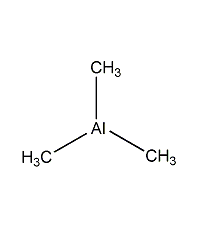
Structural formula
| Business number | 01J9 |
|---|---|
| Molecular formula | C3H9Al |
| Molecular weight | 72.09 |
| label |
Aluminum trimethanide, TMA, High purity substances and reagents, Electronic specialty gas raw materials and intermediates |
Numbering system
CAS number:75-24-1
MDL number:MFCD00008252
EINECS number:200-853-0
RTECS number:BD2204000
BRN number:3587197
PubChem number:24855575
Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 20?): 0.688
3. Relative vapor density (g/mL, air=1 ): Undetermined
4. Melting point (ºC): 15
5. Boiling point (ºC, normal pressure): 126
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºF): 40
9. Specific rotation (º ): Undetermined
10. Autoignition point or ignition temperature (ºC): -22
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC) not determined:
13. Heat of combustion (KJ/mol): not determined
14. Critical temperature (ºC): not determined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): 1.1
18. Explosion lower limit (%, V/V): 7.5
19. Solubility: reacts strongly with water
Toxicological data
Harmful if ingested, inhaled or absorbed through the skin. It has a strong irritating effect on eyes, skin and mucous membranes. Inhaling smoke particles can cause replacement of blood proteins, leading to a hyperthermia reaction.
Ecological data
It is extremely harmful to water, even in small amounts, it is toxic to fish and plankton in water bodies.
Toxic to organic matter in water.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 8
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Decomposes with moisture, air, oxides, alcohols, halocarbons, acids, bases, halides, ammonia and amines.
Storage method
Seal and store in a cool, dry, ventilated place, anti-static.
Store away from water, heat, halocarbons, alcohol, and amines.
Avoid contact with air and water.
Synthesis method
1. This product can be produced by heating metallic aluminum and dimethylmercury; or by producing trihalotrimethyldisaluminum from metallic aluminum and halogenated methane, and then reacting with metallic sodium.
2.Use Zn, EtBr and EtI to react in the presence of Cu salt and catalyst to generate EtZnX (X=Br, I), heat it to It is converted into diethyl zinc, which can be separated and purified by distillation.
3.Aluminum powder reacts with methyl iodide to form methylaluminum iodide, which is alloyed with potassium and sodium in dry decane liquid The reaction produces crude trimethylaluminum. The mixture of crude trimethylaluminum and decane is distilled under reduced pressure or normal pressure to obtain a high purity product.
Purpose
1. Trimethylaluminum is used as an olefin polymerization catalyst and ignition fuel. It is also used to prepare linear primary alcohols and olefins. It can be used for vapor deposition of metal organic compounds.
2.Used as P-type of AsGa, GaP and GaAsP when manufacturing light-emitting diodes Dopants. It is also a raw material for the MOCVD process and is used to deposit zinc films.
3.High-purity trimethylaluminum is used as a raw material for the MOCVD process to deposit aluminum films. It can also be used as a catalyst for olefin polymerization.
extended-reading:https://www.bdmaee.net/niax-a-30-foaming-catalyst-momentive/extended-reading:https://www.morpholine.org/dimethomorph/extended-reading:https://www.newtopchem.com/archives/category/products/page/5extended-reading:https://www.newtopchem.com/archives/43957extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/115.jpgextended-reading:https://www.morpholine.org/polyurethane-catalyst-1028/extended-reading:https://www.bdmaee.net/dabco-xd-102-dabco-amine-catalyst-amine-catalyst/extended-reading:https://www.newtopchem.com/archives/44365extended-reading:https://www.newtopchem.com/archives/44919extended-reading:https://www.newtopchem.com/archives/1120