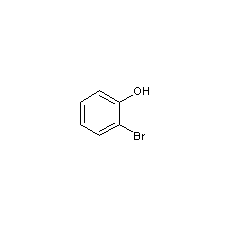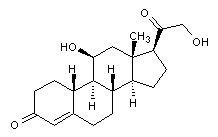
Structural formula
| Business number | 015F |
|---|---|
| Molecular formula | C21H28O5 |
| Molecular weight | 360.44 |
| label |
aldosterone, aldosterone, aldosterone, 11?,21-Dihydroxy-3,20-dioxo-4-pregnen-18-al, 11?,21-Dihydroxypregn-4-ene-3,18,20-trione, 18-Aldocorticosterone, Reichstein X, Electrocortin, 4-Pregnen-18-al-11?,21-diol-3,20-dione |
Numbering system
CAS number:52-39-1
MDL number:MFCD00051136
EINECS number:200-139-9
RTECS number:TU4523000
BRN number:3224996
PubChem ID:None
Physical property data
1. Properties: The hydrated substance is colorless crystal.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 108~112? (the melting point of anhydrous is 164?). 5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/ mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water ( Log value of the partition coefficient (octanol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/ V): Undetermined
19. Solubility: Soluble in ethanol, ether and chloroform, slightly soluble in water.
Toxicological data
1. Mutagenicity: Mutation test system – not other specifiedTEST system: non-mammalian cells – not otherwise specified: 70nmol/L; Rat intravenous DNA damage test: 23mg/kg; DNA synthesis test: 23mg/kg
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Hydrogen bond acceptorNumber: 5
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 20
6. Topological molecule polar surface area 91.7
7. Number of heavy atoms: 26
8. Surface charge: 0
9. Complexity: 682
10. Isotopes Number of atoms: 0
11. Determine the number of atomic stereocenters: 7
12. Uncertain number of atomic stereocenters: 0
13. Determine chemical bonds Number of stereocenters: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Seal and store at 0-4ºC.
Synthesis method
None yet
Purpose
1. Biochemical research. Induces the urinary excretion of potassium ions and the kidneys to absorb sodium ions.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dimethyl-tin-oxide-2273-45-2-CAS2273-45-2-Dimethyltin-oxide.pdfextended-reading:https://www.bdmaee.net/n-dimethylpropylamine/extended-reading:https://www.bdmaee.net/niax-a-133-tertiary-amine-catalyst-momentive/extended-reading:https://www.newtopchem.com/archives/40573extended-reading:https://www.newtopchem.com/archives/category/products/page/128extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/stannous-octoate-CAS-301-10-0–T-9.pdfextended-reading:https://www.newtopchem.com/archives/44245extended-reading:https://www.bdmaee.net/pc-cat-tka30-catalyst-nitro/extended-reading:https://www.bdmaee.net/dabco-t-catalyst-cas10294-43-5-evonik-germany/extended-reading:https://www.cyclohexylamine.net/reaction-delay-catalyst-polycat-sa-102-delay-catalyst-polycat-sa-102/