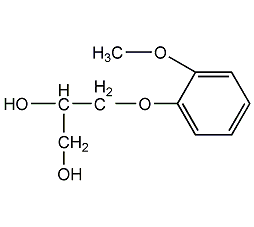
Structural formula
| Business number | 025V |
|---|---|
| Molecular formula | C10H14O4 |
| Molecular weight | 198.22 |
| label |
Guaifenesin, 3-(o-methoxyphenoxy)-1,2-propanediol, Glyceryl Guaiac, Glyceryl guaiacol, Guaifenesin, 3-(2-Methoxyphenosy)-1,2-propamediol, Aeronesin |
Numbering system
CAS number:93-14-1
MDL number:MFCD00016873
EINECS number:202-222-5
RTECS number:TY8400000
BRN number:2049375
PubChem number:24895219
Physical property data
1. Properties: colorless flaky crystals.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 76?78
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 80kPa): 147.7
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether and benzene, slightly soluble In petroleum ether, insoluble in water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 51.90
2. Molar volume (cm3/mol): 165.8
3. Isotonic specific volume (90.2K ): 431.0
4. Surface tension (dyne/cm): 45.6
5. Polarizability (10-24cm3): 20.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6.?The polar surface area of ??the molecule is 58.9
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 151
p>
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 1
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be kept sealed and dry.
Synthesis method
Originated from the condensation of sodium guaiacol and 3-chloropropanediol. Prepare sodium hydroxide solution and guaiacol at 40°C to form sodium phenate, add 3-chloro-1,2-propanediol, naturally raise the temperature to 95°C, react at 92-98°C for 3 hours, let it stand, and remove the salt water layer. , add hydrochloric acid to acidify to pH 3-5, cool and crystallize, and filter. The filter cake is recrystallized with ethanol to obtain guaifenesin. The yield is about 60%.
Purpose
This product is an expectorant and antitussive medicine.
extended-reading:https://www.newtopchem.com/archives/640extended-reading:https://www.newtopchem.com/archives/915extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Jeffcat-DMP-Lupragen-N204-PC-CAT-DMP.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dimethyltin-Dichloride-CAS-753-73-1-dimethyl-tin-dichloride.pdfextended-reading:https://www.cyclohexylamine.net/acetic-acid-potassium-salt-potassium-acetate/extended-reading:https://www.cyclohexylamine.net/dabco-bl-13-niax-a-133-jeffcat-zf-24/extended-reading:https://www.morpholine.org/cas-63469-23-8/extended-reading:https://www.bdmaee.net/di-n-octyltin-dilaurate-cas3648-18-8-dotdl/extended-reading:https://www.newtopchem.com/archives/44909extended-reading:https://www.cyclohexylamine.net/semi-hard-foam-catalyst-tmr-3-hard-foam-catalyst-tmr-3/