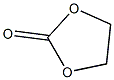
Structural formula
| Business number |
02B8 |
| Molecular formula |
C3H4O3 |
| Molecular weight |
88.06 |
| label |
1,3-dioxolane-2-one,
1,3-dioxolone,
1,2-Ethanediolcarbonate,
1,3-Dioxalan-2-one,
Glycol carbonate,
foaming agent,
Extracting agent
|
Numbering system
CAS number:96-49-1
MDL number:MFCD00005382
EINECS number:202-510-0
RTECS number:FF9550000
BRN number:106249
PubChem number:24877564
Physical property data
1. Properties: Colorless and odorless needle-like or flaky crystals at room temperature.
2. Boiling point (ºC, 101.3kPa): 248
3. Melting point (ºC): 36
4. Relative density (g/mL, 25 /4ºC): 1.3208
5. Relative vapor density (g/mL, air=1): 3.04
6. Refractive index (40ºC): 1.4199
7. Refractive index (n50D): 1.4148
8. Viscosity (mPa·s, 40ºC) : 1.92
9. Viscosity (mPa·s, 60ºC): 1.42
10. Flash point (ºC): 160
11. Fire point (ºC) : 465
12. Heat of evaporation (KJ/mol): 50.2
13. Heat of fusion (KJ/mol): 10.05
14. Heat of combustion ( KJ/mol): 834.0
15. Specific heat capacity (KJ/(kg·K), 100ºC, constant pressure): 1.93
16. Boiling point rise constant: 0.043
17. Conductivity (S/m): <1×10-7
18. Vapor pressure (kPa, 36.4ºC): 0.0027
19. Solubility: Miscible with hot water (40°C), alcohol, benzene, chloroform, ethyl acetate, acetic acid, etc. Insoluble in dry ether, carbon disulfide, carbon tetrachloride, petroleum ether, etc.
20. Refractive index at room temperature (n25): 1.415850
21. Refractive index at room temperature (n 20): 1.419540
22. Relative density (25?, 4?): 1.320840
Toxicological data
1. Acute toxicity: rat oral LD50: 10g/kg; rabbit oral LD50: >3000mg/kg; rabbit oral LD50: 10.4g/kg.
2. Animal tests have proven that it has low toxicity and is irritating to skin and eyes. Rats inhaled concentrated vapor for 8 hours without death.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 17.17
2. Molar volume (cm3/mol): 67.3
3. Isotonic specific volume (90.2K ): 166.3
4. Surface tension (dyne/cm): 37.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 6.80
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 35.5
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 60.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants, acids, and alkalis. It is a flammable liquid, please pay attention to the source of fire. Not corrosive to copper, mild steel, stainless steel or aluminum.
2. Chemical properties: relatively stable, alkali can accelerate its hydrolysis, while acid has no promoting effect on hydrolysis. In the presence of metal oxides, silica gel, and activated carbon, it decomposes at 200°C to generate carbon dioxide and ethylene oxide. When reacting with phenol, carboxylic acid, and amine, ?-hydroxyethyl ether, ?-hydroxyethyl ester, and ?-hydroxyethyl ethyl carbamate are generated respectively. Boiled with alkali to form carbonate. Ethylene glycol carbonate is heated at high temperature using a base as a catalyst to generate polyethylene oxide. Under the action of sodium methoxide, sodium monomethyl carbonate is generated. Dissolve ethylene glycol carbonate in concentrated hydrobromic acid and heat it at 100°C for several hours in a sealed tube to decompose into carbon dioxide and vinyl bromide.
3. Exist in smoke.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of ethylene oxide and carbon dioxide.
Refining method: Distillation under reduced pressure, the distillate is dissolved in ether, cooled, crystallized, filtered, and then recrystallized with anhydrous ether to obtain the pure product.
2.Add 800 ml (6.6 mol) into a three-neck flask equipped with a stirrer and radium separation columnDiethyl carbonate and 335 ml (6.0 mol) ethylene glycol, heat and stir until the temperature of the reaction solution reaches 100-105°C and the two liquid layers are miscible with each other. Remove the stirrer and add 0.3 g Anhydrous potassium carbonate and some broken magnetic pieces are heated to steam out ethanol. The temperature at the top of the separation column should not exceed 80°C, and about 98% of the theoretical amount of ethanol can be steamed out. Cool, dissolve the distillation residue in 300 ml of absolute ethanol, filter, fully cool in an ice bath, filter again, and wash the crystals with ether. Recrystallize anhydrous ethyl alcohol and dry it in a vacuum dryer containing phosphorus pentoxide to obtain the product, with a yield of 51 to 55%.
Purpose
1. Currently it is mainly used in three aspects. (1) Used as water glass-based slurry to prepare pollution-free water glass-based slurry using 1,3-dioxolane and potassium bicarbonate as curing agent and quick-setting agent respectively. Due to the acrylamide and urea system Slurries are banned, and water glass-based slurries receive widespread attention. (2) Used to synthesize furazolidone (C8H7N3O5, [67-45-8]; this is a broad-spectrum antibacterial drug used to prevent coccidiosis in chickens. (3) Used as fiber finishing agent and other processing aids. Used in fertilizers, fibers, pharmaceuticals and organic synthesis, etc.
2. Used as a solvent for nylon, polyester, polyacrylonitrile, etc., a foaming agent for plastics and rubber, a stabilizer for synthetic lubricants, and Raw material for preparing drugs, carbonates, glycidyl, etc. It is also used to selectively extract aromatic hydrocarbons from mixtures of non-aromatic hydrocarbons.
extended-reading:https://www.bdmaee.net/dibutyltin-dibenzoate-cas1067-33-0-dibutyltin-dibenzoate-solution/extended-reading:https://www.bdmaee.net/dabco-ne210-catalyst-cas10861-07-1-evonik-germany/extended-reading:https://www.morpholine.org/127-08-2/extended-reading:https://www.morpholine.org/polyester-sponge-special-catalyst-sponge-catalyst-dabco-ncm/extended-reading:https://www.newtopchem.com/archives/44031extended-reading:https://www.bdmaee.net/pc-cat-tko-catalyst-nitro/extended-reading:https://www.cyclohexylamine.net/tmr-2-cas-62314-25-4-2-hydroxypropyltrimethylammoniumformate/extended-reading:https://www.newtopchem.com/archives/44613extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/05/Lupragen-N205-MSDS.pdfextended-reading:https://www.newtopchem.com/archives/39838